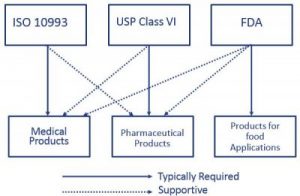
usp class vi vs iso 10993
These international standards refer to the testing requirements for bio-compatibility most commonly used in the medical sector and meet very high standards of manufacture and safety. A rubber compound has set physical parameters it needs to meet.

Material Selection Medical Injection Molding Xcentric Mold
That said the lack of risk assessment in USP Class VI can be a problem.

. May 1 2009. Iso 10993 vs. Class VI and ISO 10993 are recommendations for testing based on the use of the final device.
Inside Rubber Magazine Profiles The Rubber Group June 28 2022. Does your medical molding application require biocompatible rubber. In an effort to standardize biocompatibility testing worldwide the International Standards Organization ISO developed ISO 10993.
If yes to the first question then USP Class VI is not a relevant qualification for it. USP Class VI vs. How to Prevent Supply Chain Interruptions.
3D printing of dental and orthopedic surgical guides. USP class qualification was a key method for establishing material biocompatibility at least as far back as 1976 until the 1987 adoption of the Tripartitite Agreement. While some of our rubbers can achieve this it is important to understand the customers explicit requirements.
Tripartite introduced the first expectations of biocompatibility testing specifically focused on device-related material. Both ISO 10993 and USP Class VI define testing requirements for biocompatibility the ability of a material to perform a desired function without causing adverse effects on the human body. A more rigorous standard for the biological evaluation of medical devices is ISO-10993.
For most patient-contact applications a material that meets US Pharmacopeia USP Class VI andor ISO 109933 will be required. That said the lack of risk assessment in USP Class VI can be a problem. The first part of the ISO 10993 standard Biological Evaluation of Medical Devices.
Unlike other rubber standards theres no one standard that engineers use for an approval. Its possible that a USP Class VI material can also. USP class VI versus ISO 10993.
Though not a limited series of tests some biocompatibility requirements for medical devices may exceed the testing performed in USP Class VI. Though not a limited series of tests some biocompatibility requirements for medical devices may exceed the testing performed in USP Class VI. Take an ASTM D2000 call out.
ISO-10993 is a standard that utilizes systemic toxicity and intracutaneous reactivity testing. USP Class I II - Raw material supplier liability and responsibility. Depending on the devices use the sterilization process might.
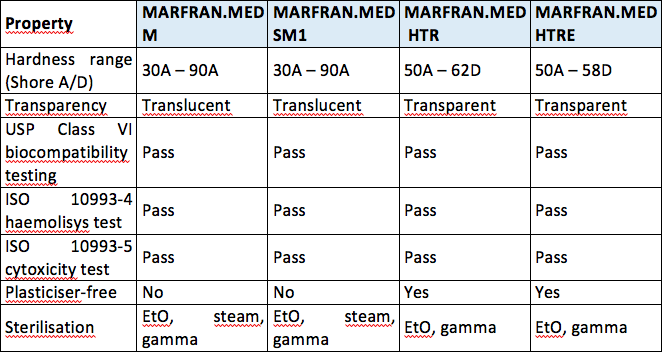
The most stringent Class VI requires three types of tests. Biocompatibility - USP Class VI vs. Typical physical properties of C-Flex Property ASTM Method Formulations Value or Rating.
A selection of Figure 4 VisiJet Accura and DuraForm plastic materials have met the requirements of ISO 10993-5 -10 or USP Class VI testing. As an example usp class vi requires an intracutaneous irritation test which is also required for iso 10993 compliance. ISO 10993 is designed for medical products that remain permanently or for a very long time in the human body so for shorter applications a USP Class VI or even a lower USP Class certification is often sufficient.
USP Class VI and ISO 10993. So does ISO 10993. USP Class VI demands an intracutaneous irritation test.
Food Grade or USP Class IV Materials for Manufacturing Injectable Products. Most applications are fairly benign to elastomers. You might establish biocompatibility via making the device of a Recognized Consensus Standard material using a validated process that does not degrade that material or by ISO 10993 testing.
USP Class VI ISO 10993-5 Cytotoxicity In-Vitro ISO 10993-3 Ames Genotoxicity ISO 10993-11 Systemic Toxicity In-Vivo ISO 10993-4 Hemolysis Indirect European Pharmacopeia 329. The materials listed below are ideal for. Rob Pruyn August 5 2020 Custom Products Medical Devices Molding Services.
Then you need to understand the differences. ISO 134852016 - Medical Device Quality Management Systems. Iso 10993 is designed for medical products that remain permanently or for a very long time in the human body so for shorter applications a usp class vi or even a lower usp class certification is often sufficient.
A number of our plastic materials are ISO-10993 or USP Class VI capable. Typically the terms USP Class VI or ISO 10993 materials are used. USP Class VI Testing is only one standard of biocompatibility however.
For this reason the FDA provides a standard 21 CFR1772600 defining allowable rubber compound ingredients and extractibles based on toxicity and carcinogenicity. Other Medical Device Regulations World-Wide. Below youll find a list of all posts that have been tagged as USP Class VI ISO 10993 vs.
Medical Molding and Biocompatible Rubber. However Class VI also requires subacute toxicity and implantation effects which many ISO 10993 categories do not. The Right Rheometer for Your Molded Rubber Parts March 30 2022.
A more rigorous standard for the biological evaluation of medical devices is ISO-10993. ISO 10993 is a 20-part standard that evaluates the effects of medical device materials on the body. Steve Melito August 5 2020.
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Advantapure Apst Silicone Tubing Translucent Platinum Cured
Brilliant Mind The World Of Tubing For Medical Use Medical Plastics News

Usp Class Vi Foster Corporation

Usp Class Vi Certification Presco Marking Products And Engineered Films
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing

Avient Corporation On Twitter Mitigating Risk And Accelerating Product Development Are Essential For Today S Medical Device Design We Can Help You With A Wide Range Of Usp Class Vi Iso 10993

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing

Bal Seal Engineering Achieves Usp Class Vi And Iso 10993 5 Compliance For Medical Sealing Polymers Bal Seal Engineering

Medical Grade Cyanoacrylate Super Glue Iso 10993 And Usp Class Vi

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group
Material Selection Medical Injection Molding Xcentric Mold

Pre Colored Medical Abs Compounds For Laser Marking Plastics Technology



